The FDA Chose Not to Ban Breast Implants Linked to Cancer and Autoimmune Problems
Latest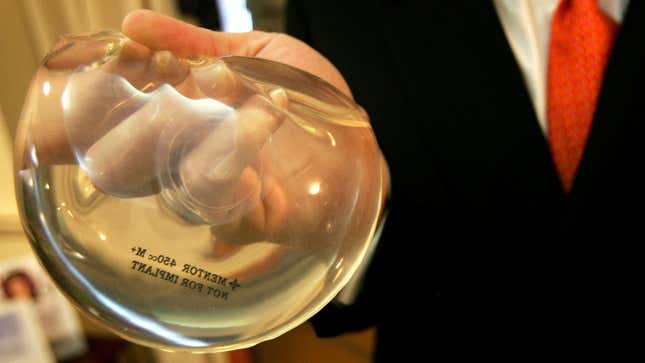

One month after hearing from dozens of women who claimed they’d suffered from health problems linked to textured breast implants, including cancer and autoimmune issues, the FDA has decided not to ban the implants.
According to The Washington Post, a statement from the FDA says the organization, “does not believe that the product — a kind of textured implant — meets the legal standard for being banned at this time, based on available data and information.”
-

-

-

-

-

-

-

-

-

-

-

-

-

-

-

-

-

-

-

-

-

-

-

-

-

-

-

-

-

-

-

-

-

-

-

-

-

-

-

-








































