When Should Women Start Getting Mammograms, and Why Is It So Controversial to Ask?
Latest

There’s an active debate in the medical community about the age at which women should start regularly going in for mammograms. In 2009, the US Preventative Services Task Force raised the starting age of its recommendation, suggesting that women receive a mammogram every other year starting at age 50 and stopping at age 74. The American Academy of Family Physicians agreed. This contradicted the recommendation of the American College of Obstetricians and Gynecologists, which recommends women receive a mammogram every year starting at age 40; the American Cancer Society co-signs age 40 as the proper start of regular screening, as does the American College of Radiology.
The reaction to the USPSTF and the AAFP’s recommendation was strong, as if the organizations had argued simply that women don’t need mammograms. That, of course, wasn’t the case: they argued, rather, that women under 50, with no family history of breast cancer or other significant risk factors, should speak to their doctors about the relative risks and benefits of mammography before undergoing screening. (Implicit is the idea that you should also have your doctor clarify whether or not you fall into this group; the default should never be to forego a recommended procedure or screening.)
But challenging anything about the conventional wisdom surrounding mammograms—even suggesting that a screening program has corollary ways of potentially causing harm—is a quick way to make people very, very angry. The logic supporting regular mammograms seems easy: breast cancer is terrible, finding it early is good. Screening allows us to find cancers before they’re otherwise clinically significant (palpable lumps, skin changes, nipple discharge). Finding these cancers earlier allows treatment of them earlier, therefore stopping more breast cancers before they metastasize, and saving more lives.
There are some huge problems hiding behind this clean logic. The benefits of mammography aren’t as great as we’d hoped. And the fact that the issue is still debated is instructive in itself. The science involved in screening decisions is far more complicated than it seems, and there’s a very real argument to be made that mammograms, for some women in some age groups, are not a good decision.
Mammography studies are contradictory. Some of the largest and most widely cited had design and implementation flaws. Some1,2,3 think those flaws don’t change the conclusions appreciably, others4 think they discredit entire studies and anyone who ever mentions them. (Those saying the flaws discredit the studies are generally on the side of the OBGYNs, the radiologists, and those pushing for earlier, more frequent screening. Those thinking they don’t change the conclusions are generally on the side of pushing the recommended screening age back to 50.)
“For context,” writes Dr. H. Gilbert Welch of Dartmouth Medical School, in a 2010 New England Journal of Medicine article, “one trial involving fewer than 150 men who were followed for less than 2 years was sufficient to convince physicians of the value of treating severe hypertension.” He adds, “That physicians are still debating the relative merits of screening mammography despite the wealth of data suggests that the test is surely a close call, a delicate balance between modest benefit and modest harm.”
In 2014, the Swiss Medical Board was charged with reviewing available data and recommending a course of action for their nation’s screening program. This board was composed of a medical ethicist, clinical epidemiologist, clinical pharmacologist, oncologic surgeon, nurse scientist, lawyer, and health economist. They raised concerns about limited benefits of mammography, potential harms of mammography, and rampant misunderstanding of the current data by both patients and physicians.
“It is easy to promote mammography screening if the majority of women believe that it prevents or reduces the risk of getting breast cancer and saves many lives through early detection of aggressive tumors. We would be in favor of mammography screening if these beliefs were valid. Unfortunately, they are not, and we believe that women need to be told so,” writes Dr. Biller-Andorno. They determined no new screening programs should be put in place, old programs should be phased out, and the public should be clearly educated as to the realistic benefits and harms of mammography screening.
In the year since then, people have remained incensed, and the debate rages. Scientific journals are rarely vitriolic, but some papers I read while researching veered perilously close to personal attacks. It’s up to women to make decisions about their own health care, but clear and up-to-date information is necessary for those decisions to be informed. And so, let’s take a closer look at the challenge to the recommendation that yearly mammograms start at age 40.
Limited Benefits
Lead-time bias
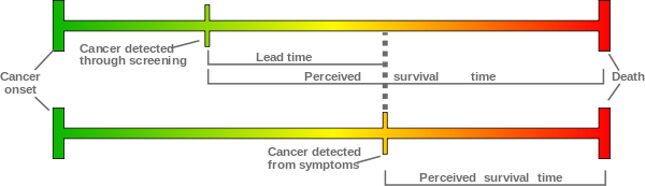
Lead-time bias is a well-known effect of any advance in testing techniques, and with breast cancer, is most simply explained like this: Let’s say we have two hypothetically identical patients. Both develop identical breast cancers at the age of 45. Without screening, patient A’s cancer is discovered at 50 and she tragically dies at 55. With screening, patient B’s cancer is discovered at 45 and she tragically dies at 55. In this example, screening had no effect on survival. The two women had the exact same outcome, but the screened patient looks to have survived double the length of time after diagnosis (10 versus five years).
Lead-time bias is generally controlled for during implementation of new screening processes, and alone isn’t a source of controversy in the mammography argument. The researchers involved in these studies expected and accounted for lead-time bias. Careful analysis of prevalence (the number of cases of disease in a given population), incidence (the number of new cases of disease in a given population), and survival rates allow us to parse the real benefits.
But lead-time bias is relevant to this discussion, as it intersects with other factors. Like:
Breast-cancer awareness
Awareness is great. Prevention is great. There’s nothing more important to an individual’s health than their own education and seeing a primary care physician routinely. Increasing the public’s awareness of the rampant and significant threat posed by breast cancer is vitally important.
It also confuses the data. As more women are aware and pursuing treatment for breast changes, we’ll find more cancers and, conceivably, find them earlier. This could change both incidence and prevalence at any specific point in time. Ironically, the massive push toward educating the public about breast cancer and encouraging widespread mammogram screening (specifically in the ‘80s) affected these totals, and further complicated analysis from studies specifically designed to measure the effectiveness of said screening.

Advances in treatment
Medicine is constantly evolving, and it’s a physician’s responsibility to both keep constantly abreast (ha) of the current standards of care and evolving treatments, as well as professionally and expertly balance the risk of comparatively new, untested treatments with the tried-and-true gold standards. A screening process like mammograms adds another layer of complexity (again). As we’ve gotten better at successfully treating breast cancer, the importance of early detection can change.
Ultimately, the single population that benefits from screening is: women with breast cancer which would prove devastating if not detected early through mammography. This population, due to advances in treatment, isn’t static. While advancements certainly haven’t nullified benefits from early detection (we’re not that good yet), the number of cases fitting this particular population changes as our ability to treat detected cancers improves.
A Norwegian study followed all women ages of 50-79 between the years 1986-20098 (“all women” meaning all the women in the country; there are some real academic advantages to a national health registry). Norway implemented their national mammography screening program between the years 1995-2005. They utilized a staggered implementation, which allows for identifying benefits independent of lead-time bias or changes in breast cancer awareness. This screening process involved both mammograms and multidisciplinary teams managing treatment of detected cancers.
In this study, there was a population that received only multidisciplinary team care but not mammograms (allowing separate comparison of mammogram benefit, interdisciplinary team benefit, and combined benefit). They found a 10 percent decrease in breast-cancer-related deaths to patients receiving both care modalities. Those receiving just interdisciplinary team care but no mammography screening showed an 8 percent decrease in breast-cancer-related deaths. This implies only a 2 percent reduction in deaths due to mammography alone.
And in reality, the incidence of breast cancer mortality is quite low. Dr. Welch, from the earlier New England Journal of Medicine article, points out that the “10-year risk of breast-cancer death for a 50-year-old woman in the United States is now about 4 per 1000 women…if we assume that this risk already incorporates the benefit of screening mammography, the risk estimate without mammography would be about 4.4 per 1000 women.”1
An important idea here is “number needed to treat.” Using incidence, prevalence, and survival rates with treatment, this number estimates the number of otherwise healthy women who would need to be screened in order to save one life. If screening only saves .4 lives per 1000 screened, then “2500 women would need to be screened over a 10-year-period for 1 to avoid death from breast cancer.”1
“Less benefit” is still a benefit, of course; that one life saved is worth any amount of screening, many would say. But screening isn’t an absolutely harmless process. The 2499 women mentioned above who didn’t benefit—is it possible that some would be worse off?
Potential Harm
False positives
This refers to mammography findings that warrant further workup and eventually prove benign. False positives occur with every test: PSA screening, rapid strep-throat swabs, HIV testing, etc. The rates within mammography findings vary by region, as they’ll largely be driven by radiologist interpretation. This isn’t to say that some radiologists are right and some are wrong, but just that no two individuals have identical thresholds at which they’ll state a mammogram requires further workup.
There’s a direct trade-off here. Those with lower thresholds, who are quicker to say “this needs to be examined more,” will both miss fewer cancers (fewer false negatives) and work up more cancer-free patients (more false positives). There’s also a significant difference between European and American rates, likely due to differences in physician training and treatment modalities. From a 2015 Breast Cancer Research study:
“The risk of experiencing a false positive mammogram for women undergoing biennial screening from age 50-69 years in Europe is about 20%, and the risk of experiencing a biopsy due to a false positive test is 3%…The risk is even higher in the US, where the 10-year false positive rate is 30%, and 50% of all women will experience a false positive mammogram at one time.”
So, who cares? False positives seem like an acceptable risk. They get worked up, findings are ultimately non-threatening, and everyone goes about their business. Unfortunately, the tests and procedures following a positive finding can prove far from benign. The workup following these false positives varies widely. Some options include an ultrasound, a repeat mammogram, a fine needle aspiration (what it sounds like), or a lumpectomy (also exactly what it sounds like). A New York Times article from 2014 pointed out the health risks that can accompany treatment.
Even a simple repeat mammogram is not without cost to the patient. While these are sometimes done quickly, they’re often scheduled months down the road. As you can imagine, being told “we found something suspicious, it’s probably nothing, but we’d like to repeat a mammogram in three months” does not make for a relaxing interval. Multiple studies have been conducted to try and determine the effects of such procedures on both the mental wellbeing of patients and their ongoing trust in the healthcare system. The last thing we want a screening test to do is invoke anxiety and reluctance to return to the physician.
And, again, these studies have wildly varying results.2,5,6 Findings not only differ from study to study in the same geographical area, but also between various cultures. Understanding of mammography, a culture’s specific stigmas concerning breast cancer, a patient’s understanding of cancer, and the degree of trust in the medical community will all play a role in determining how distressing pending workup can be.
Futile treatment
Some of you probably noted that the 2499 women without benefit includes both women with and without positive screening. We’ll start with those with for whom mammography detects ultimately fatal cancer. Based on our 4 per 1000 stats from earlier, that’d be roughly 10 women.
So, another hypothetical. Just like before, we have two identical women, each developing breast cancer at 45. Woman A has her cancer discovered at 50 without screening, and tragically passes away at 55 after undergoing surgery, radiation, chemotherapy, and whatever other treatments her team uses to try and cure her. Woman B has her cancer caught by screening at 45, and tragically passes at 55 after undergoing similarly invasive care. Same outcome, but one had the benefit of living from 45-50 without the burden of cancer.
It sounds like an inhumane opinion, but it’s a statistical reality that the above scenario is going to occur, and the psychological cost of a cancer diagnosis and treatment is very, very real.
Current public understanding
The findings from the Swiss medical board (the group which suggested abolishing mammography screening programs entirely) mention an important point. The public simply does not have an accurate understanding of the benefits/harms of mammography screening. The following graph compares the public’s perception with the actual observed effects.
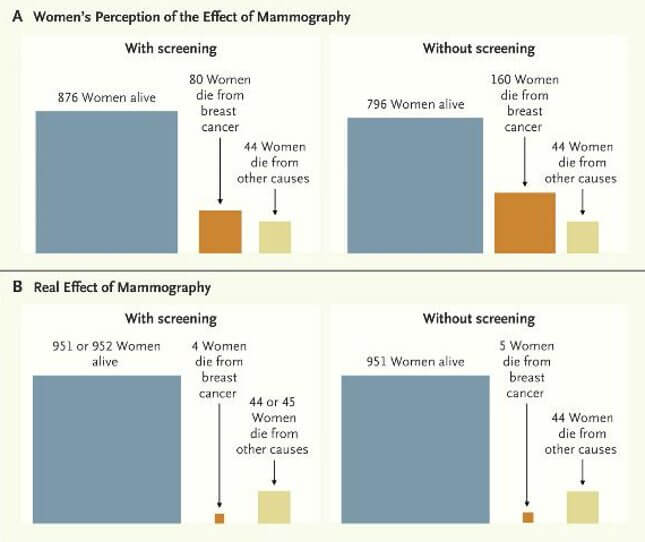
This perhaps is the most telling factor of all. Because of the assumption that mammography has a larger real effect than it actually does, it’s unsurprising that the USPSTF, the AAFP, and the Swiss board’s recommendations have all instigated a firestorm of controversy; the latter in particular was condemned as unethical and “unsettling to women.3”
But unsettling women by educating them about mammography isn’t a problem. It’s a necessity.
Overdiagnosis
This is the worst one. Overdiagnosis refers to the discovery and treatment of cancers which would have never become clinically significant. It’s a similar line of thinking to suggestions concerning mammography for women past the age of 74.
Let’s go back to our two hypothetical women. A 75-year-old woman who is otherwise healthy with an appreciably long life expectancy may still benefit from mammography. Screening and early detection could have a significant effect on the outcome of her breast cancer. Meanwhile, another 75-year-old woman has hypertension and diabetes and heart disease and all of the other things at which we Americans excel. Let’s say her life expectancy is roughly 5 years. At this point, the chances of breast cancer being the disease to kill her are very slim, making mammography screening likely not worth the risk.
There’s a parallel to draw here with prostate cancer. Prostate cancer is everywhere. It’s even been hypothesized as “the norm” when a man reaches an advanced age. It’s also, usually, a very slow-growing cancer, both slow to grow and slow to spread. Some of you likely have an older male relative who was told by his doctor, “You’ve got prostate cancer, but we’re not going to do anything. It’s not going to be what kills you.”
But, we’re not talking about women over the age of 74. We’re talking about women under 50. Clearly, this population has a longer life expectancy, and the general idea here is that some of the discovered cancers would be treated unnecessarily. This can be because the tumor wouldn’t progress, would regress, or another illness would kill the patient before the cancer became an issue. It’s impossible to differentiate between those three specific examples, and almost impossible to accurately measure the prevalence of overdiagnosis at all.
Findings vary wildly, estimating that overdiagnosis occurs anywhere from 0 to 54% of the time. From a Breast Cancer Research study: “In studies based on statistical modeling to adjust for lead-time, estimates of overdiagnosis are consistently below 5%. In contrast, observational studies have published higher estimates, between 22 and 54%.”2
In particular, observational studies use an elegantly simple analysis. With a long enough follow-up time, we’d expect screened and non-screened populations to have identical rates of invasive cancer. Screened populations would present more cancers earlier, and show higher rates of cancer at the start of the study. But as time goes on, undetected cancers in the non-screened populations would develop, be discovered, and the rates between the two groups would equalize. Any long-lasting differences in cancer rates between the two groups—and there are differences—imply overdiagnosis.
The opposing arguments
The other side—those who not only believe that mammography screening is beneficial starting at age 40, but also say that to discourage mammograms in women under 50 is to directly harm public health—is significant and not without merits.
Much of their specific outrage is directed at conclusions drawn from the Canadian National Breast Cancer Screening Study (CNBSS)7, a huge study following women over 25 years which is one of the major sources of data comprising the arguments presented above. It’s also the study I mentioned at the start, with some design flaws calling into question the results. (While there are more studies than just the CNBSS arguing against widespread mammography, many of their arguments are generalizable to the conclusions as a whole.)
Detractors note that some of the studies allowed for clinical exams before placement into screening or non-screening groups.4 This allowed selection of women with palpable breast lumps into the screening group. The thinking was, likely, that this facilitated faster detection and treatment for women more likely to have breast cancer. That makes sense, but could also artificially inflate the prevalence of breast cancer in the screening group. Remember, long-term prevalence is the key to estimating overdiagnosis, so any disruption to randomization here can confuse results 25 years down the road.
They also point out that recommended biopsies weren’t followed systematically4, potentially distorting end effects of early detection. I suspect that treatment and analysis of biopsies were left to the discretion of patients and their individual oncological teams. Once mammography has a positive finding, further treatment decisions would need to be made based on the standards of care for that specific case rather than study guidelines put in place as many as 25 years ago (remember, those standards of care and treatment modalities are constantly evolving).
Additionally, they point out that grouping analysis into “women under 40” and “women over 40” is inherently arbitrary.4 There is no change to a woman’s breast physiology, or to breast cancer pathology, at that age. No switch gets flipped. It may be that the age we should be discussing is 38 or 42 or 45.
There’s also an unpleasant addendum to this conversation that is unfair, but frequently raised. The major organizations of physicians defending breast cancer screening starting at age 40 are the American College of Obstetricians, the American Cancer Society, and the American College of Radiology. These are the physicians most actively involved in detecting and treating breast cancer. Someone invariably points out they’re also the physicians financially benefiting from said work. But these doctors aren’t pushing for a paycheck. They care deeply about their patients. They see the horribly destructive and deadly effects of breast cancer more often than the rest of us. And they see positive outcomes after screening and early detection. Their dissension and outright fury isn’t because they might see a few less mammogram workups each year. The abolition of screening programs wouldn’t leave them out of work for a single minute of a single day. They simply disagree, and think the views I’ve espoused here have the potential to do active harm.
There is one thing we all agree on. Education for both women and healthcare professionals everywhere needs to be improved on this issue. So please, if you take away anything from this article, make it that. Read the arguments. Examine the evidence. Discuss both with your physician. Ask them if they believe you should undergo mammography screening. Ask them what they think of the controversy, conflicting viewpoints, and ongoing debate. Ask why.
Illustration by Tara Jacoby. Images via Wikimedia, ACS, NEJM.
Jesse Fredeen is a 4th year medical student at Marshall University just a few short months away from his MD. He’s written for Deadspin, Sports on Earth, and VICE Sports. You can follow him on twitter @jessefredeen, which will be unprotected as soon as he’s done matching into a residency program.
Sources:
- Welch, H.G. (2010). Screening Mammography – A Long Run for a Short Slide? The New England Journal of Medicine. 363:13.
- Loberg, M., Lousdal, M.L., Bretthauer, M., Kalager, M. (2015). Benefits and harms of mammography screening. Breast Cancer Research. 17:63.
- Biller-Andorno, N., Juni, P. (2014). Abolishing Mammography Screening Programs? A View from the Swiss Medical Board. The New England Journal of Medicine. 370:21.
- Heywang-Kobrunner, S.H., Schreer, I., Hacker, A., Noftz, M.R., Katalinic, A. (2015). Conclusions for mammography screening after 25-year follow-up of the Canadian National Breast Cancer Screening Study (CNBSS). European Radiology.
- Heleno, B., Siersma, V.D., Brodersen, J. (2015). Diagnostic Invasiveness and Psychosocial Consequences of False-Positive Mammography. The Annals of Family Medicine. 13:242-249.
- Kitano, A. et al. (2015). Psychological impact of breast cancer screening in Japan. Japan Society of Clinical Oncology. May.
- Biller, A.B. et al. (2014). Twenty five year follow-up for breast cancer incidence and mortality of the Canadian National Breast Screening Study: randomised screening trial. The British Medical Journal. 2014;348:g366.
- Kalager, M. et al. (2010). Effect of Screening Mammography on Breast-Cancer Mortality in Norway. The New England Journal of Medicine. 363:1203-1210.